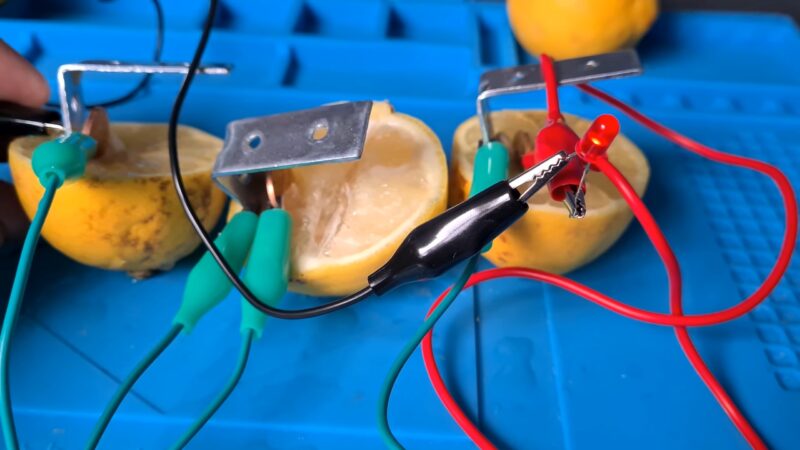
Have you ever wondered if you can power a light bulb with a lemon? Or how a lemon can act as a battery?
In this blog post, we will show you how to make a lemon battery science fair project that can harness the energy from a lemon and use it to light up an LED. This is a fun and easy experiment that you can do at home or in the classroom, and learn about the concepts of electricity, chemical reactions, and circuits.
Let’s get started!
What You Need

To make a lemon battery, you will need the following materials:
- A lemon (or any other citrus fruit)
- A zinc nail (or a galvanized nail)
- A copper coin (or a piece of copper wire)
- An LED (or a small light bulb)
- Alligator clips (or wires)
- A voltmeter (optional)
How It Works
A lemon battery is a type of electrochemical cell, which is a device that converts chemical energy into electrical energy. An electrochemical cell consists of two electrodes (the zinc nail and the copper coin) and an electrolyte (the lemon juice).
Electrochemical Processes
The electrodes function as conduits for electricity, while the electrolyte facilitates the flow of electric charge. When these components are inserted into the lemon, a chemical reaction ensues, primarily involving the zinc and the lemon juice.
As the zinc dissolves in the lemon juice, it releases negatively charged electrons. These electrons then traverse from the zinc nail to the copper coin through the external circuit, which includes components like the LED and wires.
This movement of electrons constitutes an electric current, providing the power necessary to illuminate the LED.
Unique Reactions and Limitations
Simultaneously, the copper coin undergoes a distinct reaction with the lemon juice. It accepts electrons from the lemon juice, leading to a positive charge.
The lemon juice, containing positively charged hydrogen ions, is attracted to the negatively charged copper coin. The combination of hydrogen ions and electrons results in the formation of hydrogen gas, which escapes as bubbles from the lemon.
The functionality of the lemon battery relies on a sufficient supply of zinc and lemon juice to sustain the ongoing chemical reaction. Unfortunately, as time progresses, the zinc and lemon juice are depleted, causing a reduction in electric current.
Eventually, the lemon battery exhausts its resources, resulting in a loss of power and the cessation of the LED’s operation.
How to Make It

Create a lemon battery with the following steps:
- Begin by cutting the lemon in half and giving it a slight squeeze to release some juice.
- Insert the zinc nail into one lemon half, ensuring it avoids contact with the peel.
- In the other lemon half, insert the copper coin, making sure it remains peel-free.
- Connect one end of an alligator clip to the zinc nail and the other end to the longer leg of the LED; this marks the positive terminal.
- Attach another alligator clip to the copper coin and the opposite end to the shorter LED leg; this signifies the negative terminal.
- Observe the LED to check for illumination. If it doesn’t light up, experiment by reversing the connections or trying a different LED.
How to Measure It
When evaluating the power of your lemon battery, the measurement of voltage and current is crucial. Utilize a voltmeter for voltage and an ammeter for current.
Alternatively, a multimeter, capable of measuring both, can streamline the process.
Voltage Measurement
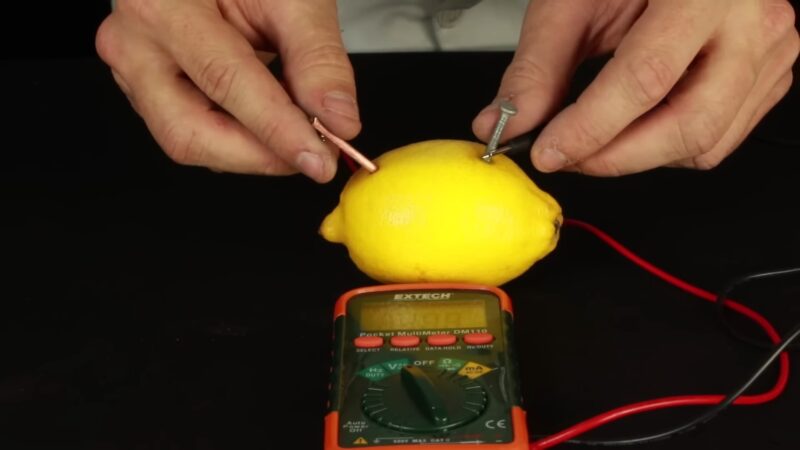
In order to check the power of your lemon battery, use a voltmeter for voltage and an ammeter for current. A voltmeter measures the electric potential difference, while an ammeter measures the current in the circuit.
You can also use a multimeter, which is a device that can measure both voltage and current. To measure the voltage of the lemon battery, follow these steps:
- Set the voltmeter (or the multimeter) to the DC voltage mode, and choose a suitable range (for example, 2 volts).
- Connect the positive probe of the voltmeter to the positive terminal of the lemon battery, and the negative probe to the negative terminal.
- Read the voltage on the display of the voltmeter. It should be around 0.9 volts, depending on the size and freshness of the lemon.
Current Measurement
To measure the current of the lemon battery, follow these steps:
- Set the ammeter (or the multimeter) to the DC current mode, and choose a suitable range (for example, 10 milliamps).
- Disconnect the alligator clip that connects the LED to the negative terminal of the lemon battery, and insert the ammeter in between. This means that the current will flow from the lemon battery to the LED, to the ammeter, and back to the lemon battery.
- Read the current on the display of the ammeter. It should be around 1 milliamp, depending on the brightness of the LED.
How to Improve It
To make your lemon battery more powerful, you can try the following ideas.
Use More Lemons
You can connect several lemons in a series, which means that you connect the positive terminal of one lemon to the negative terminal of another lemon, and so on. This will increase the voltage of the lemon battery, and make the LED brighter.
You can also connect several lemons in parallel, which means that you connect the positive terminals of all the lemons together, and the negative terminals of all the lemons together. This will increase the current of the lemon battery, and make the LED last longer.
Experiment with Various Electrodes
Explore the impact of different metals like iron, aluminum, or silver on the voltage and current of your lemon battery. Vary the shapes and sizes of electrodes, such as nails, screws, or wires, to observe how they influence the surface area and resistance of the lemon battery.
Explore Different Electrolytes
Test the effects of using various liquids, such as orange juice, vinegar, or saltwater, on the acidity and conductivity of the lemon battery. Additionally, experiment with different substances like baking soda, sugar, or bleach to observe how they influence the chemical reaction and overall performance of the lemon battery.
How to Explain It
To explain your lemon battery science fair project, you can use the following table to summarize your findings:
| Variable | Effect | Explanation |
|---|---|---|
| Number of lemons | Increased lemons in series boost voltage, while more lemons in parallel enhance current. | Adding lemons in series accumulates electric potential difference. Adding lemons in parallel increases the available electric charge for flow. |
| Type of electrodes | Diverse metals exhibit varying reactivity and conductivity. | More reactive metals release more electrons, generating higher voltage. More conductive metals facilitate increased electron flow, creating a higher current. |
| Type of electrolyte | Various liquids vary in acidity and conductivity. | More acidic liquids yield more hydrogen ions, leading to higher voltage. More conductive liquids enhance electric charge flow, resulting in higher current. |
You can also use the following diagram to illustrate how the lemon battery works:
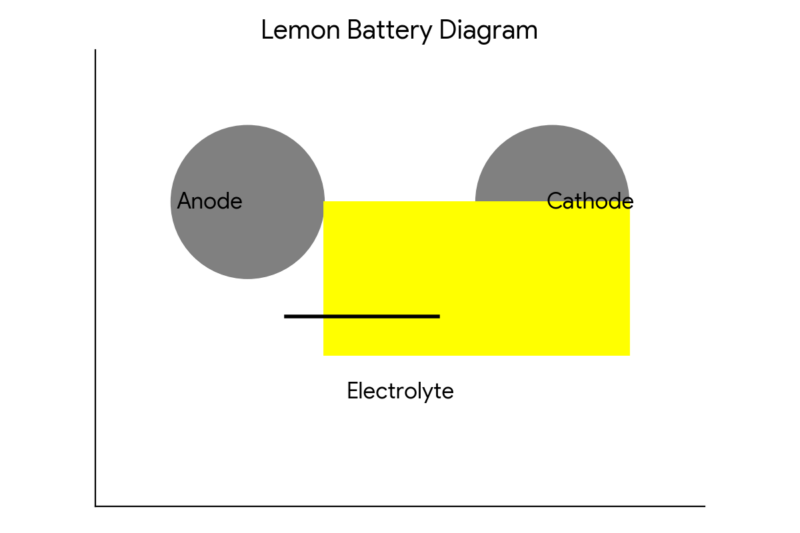
In this diagram, the zinc nail is the anode, which is the electrode where oxidation (loss of electrons) occurs. The copper coin is the cathode, which is the electrode where reduction (gain of electrons) occurs.
The lemon juice is the electrolyte, which is the medium that allows the electric charge to flow. The LED is the load, which is the device that uses electric energy.
You can also use the following equation to describe the chemical reaction that occurs in the lemon battery:
Zn(s) + 2H⁺(aq) → Zn²⁺(aq) + H₂(g) + 2e⁻
In this equation, the zinc atom loses two electrons and becomes a zinc ion, which dissolves in the lemon juice. The hydrogen ion gains two electrons and becomes a hydrogen atom, which forms hydrogen gas.
The electrons flow from the zinc nail to the copper coin through the external circuit.
FAQ
How many lemons do I need to light up an LED?
For effective LED illumination, use multiple lemons based on the LED’s type and size. Some LEDs require more voltage or current than a single lemon can deliver. Enhance the lemon battery by connecting two or more lemons in series or parallel. Experiment with larger or fresher lemons to optimize overall performance.
What are some real-life applications of lemon batteries or similar devices?
Lemon batteries serve as emergency power sources, educational tools, and environmental sensors. They power devices like clocks, radios, and calculators, aiding educators in teaching electricity and chemistry principles. Researchers use lemon batteries to measure soil or water pH levels.
Why do I need to use metals with different reactivity for the electrodes?
Choose metals with varying reactivity for electrodes to establish an electric potential difference. More reactive metals become anodes, losing electrons easily, while less reactive metals become cathodes, adept at gaining electrons. A greater reactivity difference results in a higher lemon battery voltage.
How can I test the pH level of the lemon juice or other liquids?
Assess the pH level of liquids using pH indicators like litmus paper, phenolphthalein, or red cabbage juice. Observe color changes in the indicator when dipped into the liquid, comparing them to a pH scale ranging from 0 to 14, where lower values indicate acidity, higher values denote alkalinity, and 7 represents neutrality.
What are some safety precautions to take when making a lemon battery?
Prioritize safety by wearing gloves, goggles, and aprons when crafting a lemon battery to protect against lemon juice and sharp metal edges. Minimize injury risk by avoiding direct contact with electrodes and wires. Dispose of the lemon battery responsibly to prevent leakage or corrosion.
How can I make a lemon-battery clock or a lemon-battery calculator?
Utilize the materials and steps from the lemon battery LED process to craft a lemon battery clock or calculator. Substitute the LED with the desired device and connect the lemon battery’s positive and negative terminals to the corresponding clock or calculator terminals. Adjust the lemon quantity or electrodes as needed to meet the power requirements of the specific device.
What are some other fruits or vegetables that can be used to make a battery?
Explore battery options with various fruits or vegetables such as potatoes, tomatoes, apples, oranges, grapefruits, limes, cucumbers, carrots, or onions. These items contain acids or salts acting as electrolytes, akin to lemon juice. Keep in mind that the battery’s voltage and current may fluctuate based on the type and freshness of the chosen fruit or vegetable.
Final Words
In this blog post, we have learned how to make a lemon battery science fair project that can harness the energy from a lemon and use it to light up an LED. We have also learned how to measure, improve, and explain the lemon battery.
We hope that you have enjoyed this experiment and learned something new. Have fun and stay curious!